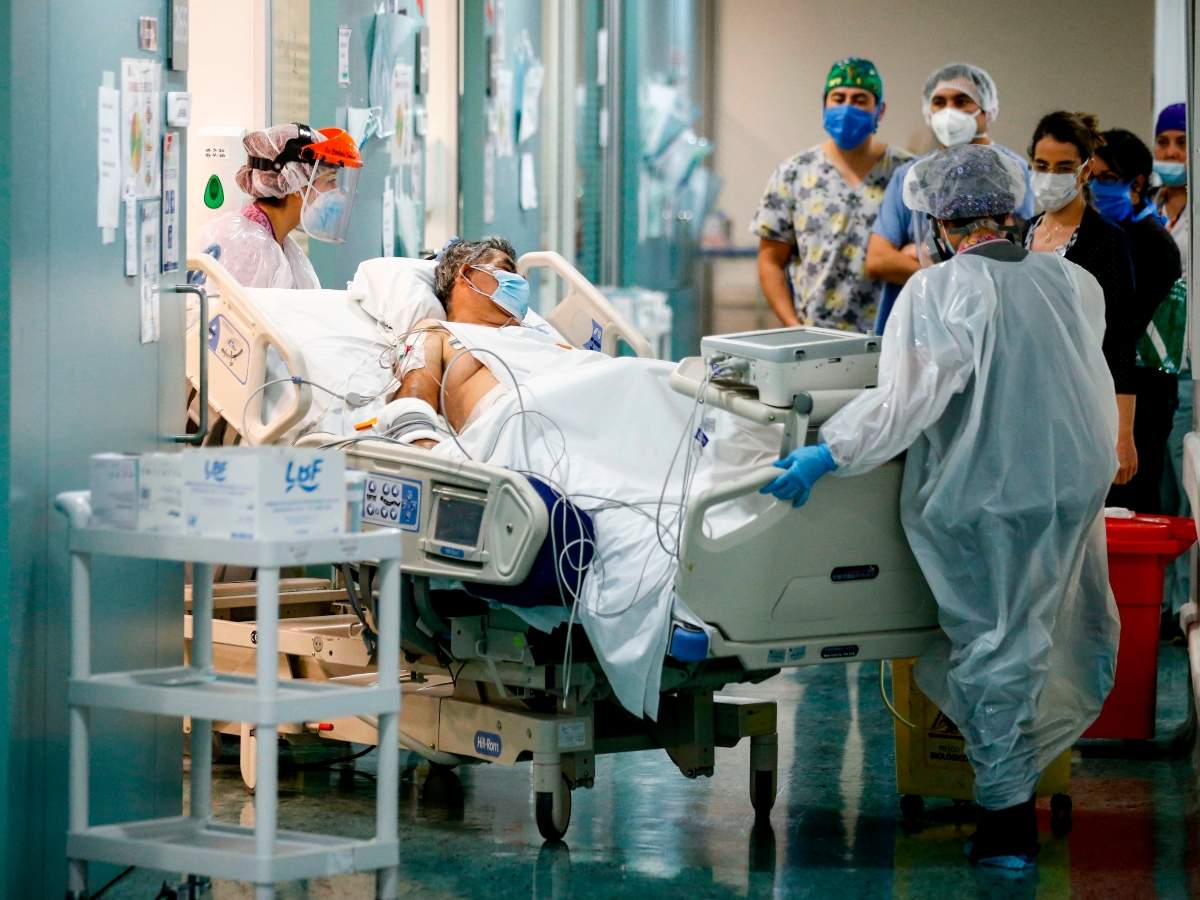
 WASHINGTON D.C: Treating coronavirus sufferers with blood thinners could assist in boosting their prospects for survival, in line with preliminary findings from physicians at New York Metropolis’s largest hospital system that gives one other supply of hope in treating the lethal an infection.
WASHINGTON D.C: Treating coronavirus sufferers with blood thinners could assist in boosting their prospects for survival, in line with preliminary findings from physicians at New York Metropolis’s largest hospital system that gives one other supply of hope in treating the lethal an infection.The Washington Publish reported that the outcomes of the evaluation, carried out over 2,733 sufferers, have been revealed on Wednesday within the Journal of the American Faculty of Cardiology.
In an interview to the newspaper, Valentin Fuster, a doctor in chief at Mount Sinai Hospital and one of many examine’s authors, stated that the observations have been based mostly solely on a assessment of medical information and that extra rigorous and randomised research are wanted to attract broader conclusions.
However the outcomes have been promising, he stated.
“My opinion is cautious, however I have to inform you I believe that is going to assist. That is the opening of the door for what medication to make use of and what inquiries to reply,” the doctor additional acknowledged.
Since March, when coronavirus hit Europe and the USA, docs have been reporting mysterious blood clots, which may be gel-like and even semisolid, in a major subset of coronavirus sufferers. Autopsies of sufferers who died of respiratory arrest have proven that some had uncommon micro clots of their lungs moderately than the everyday injury anticipated.
Final month, docs reported within the New England Journal of Medication on 5 uncommon instances of Covid-19 constructive individuals of their 30s and 40s experiencing giant strokes.
The Mount Sinai examine targeted on hospitalised sufferers who have been handled from March 14 to April 11. Among the many sufferers, those that weren’t on ventilators and handled with blood thinners died at related charges compared to those that didn’t obtain blood thinners. However the group of sufferers who obtained blood thinners lived longer — a median of 21 days in comparison with 14 days, the examine stated.
For sufferers on ventilators, the distinction was extra vital. About 63 per cent of sufferers who didn’t obtain the medicines died in contrast with 29 per cent who obtained the therapy.
One other vital discovering of the examine was that giving blood thinners to those sufferers seemed to be comparatively protected. There was not a major distinction in essentially the most harmful aspect impact of anticoagulants — bleeding — in those that have been on the medication and those that weren’t.
Fuster stated that because of the evaluation, the hospital system modified its therapy protocols a number of days in the past to start giving larger doses of blood thinners to the sufferers contaminated with Covid-19.
Deepak Bhatt, a professor at Harvard Medical Faculty who specialises in interventional cardiology, referred to as the paper “a vital examine” with the blood points in COVID-19 sufferers having developed from only a suspicion to a well-recognised complication of the virus. “What we’re figuring now’s what will we do now that we all know when it comes to remedies,” he stated.
Quite a few medical societies, together with the Worldwide Society on Thrombosis and Haemostasis and the American Society of Hematology, have put out steering recommending using blood thinners for some Covid-19 sufferers, however the recommendation has taken a conservative strategy.
“It’s a delicate steadiness between clotting and bleeding, particularly when sufferers are as sick as among the ones who’ve Covid-19,” stated Geoffrey Barnes, an assistant professor on the College of Michigan who works in cardiovascular drugs.
“Per week in the past, we have been making some educated guesses on the way to forestall blood clots. That is the primary time we now have seen information that claims larger doses could presumably be efficient and protected,” he was quoted as saying.
Docs caring for the sickest coronavirus sufferers confront a restricted arsenal of remedies. On Could 1, the Meals and Drug Administration issued an emergency use authorisation for the antiviral drug remdesivir in sufferers who’re hospitalised and significantly ailing.
However trials of different remedies, together with these involving Hydroxychloroquine, a malaria drug touted by US President Donald Trump, have been stopped due to an absence of efficacy and issues about toxicity.
In late April, scientists additionally reported that an arthritis drug made by Regeneron and Sanofi that had drawn enthusiasm from traders early on produced disappointing ends in medical trials, in line with The Washington Publish.

0 Comments